Ridgefield, Conn., U.S., and Ingelheim, Germany
- Zongertinib would be the first orally administered, targeted therapy for previously treated patients with HER2 (ERBB2)-mutant advanced non-small cell lung cancer (NSCLC), if approved
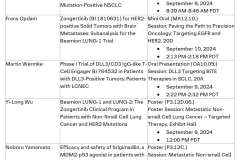
- The application for this investigational treatment is based on positive results from the Phase Ib Beamion LUNG-1, Cohort 1 trial that demonstrated an objective response rate of 71% in 75 previously treated patients with advanced NSCLC
- HER2 (ERBB2)-mutant advanced NSCLC is linked to poor prognosis and currently has limited treatment options1
Boehringer Ingelheim today announced that the U.S. Food and Drug Administration (FDA) has granted Priority Review to its new drug application for zongertinib (BI 1810631) for the treatment of adult patients with unresectable or metastatic non-small cell lung cancer (NSCLC) whose tumors have HER2 (ERBB2) mutations and who have received prior systemic therapy. The FDA grants Priority Review to applications for drugs that would offer significant improvements in the treatment, diagnosis, or prevention of serious conditions, with action expected within six months compared to 10 months under standard review. The Prescription Drug User Fee Act (PDUFA) action date is in the third quarter of 2025.
“We believe zongertinib has the potential to transform the care of previously treated patients with HER2 (ERBB2)-mutant advanced non-small cell lung cancer and are hopeful about the continued research in other tumor types and lines of therapy,” said Shashank Deshpande, Member of the Board of Managing Directors and Head of Human Pharma at Boehringer Ingelheim. “Priority Review illustrates the urgent need in this patient population and the possibility for zongertinib to be a groundbreaking innovation for patients with limited treatment options.”
The application was based on results from the positive Phase Ib Beamion LUNG-1 trial. Data from Cohort 1 (N=75) of the study, demonstrated an objective response rate (ORR) of 71% with six-month progression-free survival (PFS) and duration of response (DoR) rates of 69% and 73%, respectively, in patients with mutations in the HER2 tyrosine kinase domain.
Zongertinib had a safety profile with a low incidence of dose reductions (5%) and treatment discontinuations (3%). The majority of treatment-related adverse events (TRAEs) with zongertinib were mild in nature with diarrhea and rash being the most common all grade TRAEs, at 51% and 27% respectively. No new safety signals were observed. Grade 3 or higher TRAEs occurred in one patient treated with zongertinib. No treatment-related interstitial lung disease (ILD) cases were reported.
“Personalized medicine has revolutionized cancer treatment,” said GO2 for Lung Cancer’s Chief Scientific Officer, Courtney Granville. “Early screening and biomarker testing for mutations provide critical information to guide targeted therapies in personalized medicine. This filing acceptance represents a significant step toward offering another option for individuals with a HER2 (ERBB2) diagnosis, bringing hope and direction to cancer patients.”
Zongertinib was previously granted Breakthrough Therapy Designation and Fast Track Designation by the FDA. The FDA’s Breakthrough Therapy designation is intended to expedite the development and review of a medicine that is intended to treat a serious or life-threatening disease, and preliminary clinical evidence indicates the drug may demonstrate substantial improvement over available treatments. The FDA's Fast Track program is designed to facilitate the development and expedite the review of drugs to treat serious conditions and fill an unmet medical need. In addition to the FDA designations, Japan’s Pharmaceuticals and Medical Devices Agency recently granted Orphan Drug Designation to zongertinib.
About the Beamion clinical trial program
Beamion LUNG-1 (NCT04886804) is an open-label, Phase I dose escalation trial, with dose confirmation and expansion, of zongertinib as monotherapy in people with unresectable or metastatic solid tumors with HER2 (ERBB2) alterations. Beamion LUNG-2 is a Phase III, open label, randomized, active-controlled study that is enrolling patients with unresectable or metastatic non-squamous non-small cell lung cancer (NSCLC) harboring HER2 (ERBB2) tyrosine kinase domain mutations to evaluate zongertinib compared with standard of care.
About zongertinib
Zongertinib (also known as BI 1810631) is an investigational, irreversible tyrosine kinase inhibitor (TKI) that selectively inhibits HER2 (ERBB2) while sparing EGFR, thereby limiting associated toxicities. This orally administered, targeted treatment is being developed for HER2 (ERRB2)-mutant advanced non-small cell lung cancer (NSCLC) and additional clinical studies with zongertinib are ongoing in solid tumors with HER2 alterations.
About non-small cell lung cancer (NSCLC)
Lung cancer claims more lives than any other cancer type and the incidence is set to increase to over 3 million cases worldwide by 2040.2 NSCLC is the most common type of lung cancer.3 Due to a lack of symptoms and misdiagnoses,4 most patients diagnosed with NSCLC present at stage III or IV, where the disease has metastasized locally or to other organs.5 Fewer than 3 in 10 patients are alive five years after a diagnosis of HER2 (ERBB2)-mutant advanced NSCLC.6 People living with advanced NSCLC can experience a detrimental physical, psychological, and emotional impact on their daily lives. There remains a high unmet need for additional treatment options for people living with advanced NSCLC. HER2 (ERBB2) mutations occur in approximately 2–4% of NSCLC cases and are associated with a poor prognosis and higher incidence of brain metastases.7,8 Mutations in HER2 (ERBB2) can lead to overexpression and overactivation, which can in turn result in uncontrolled cell production, inhibition of cell death and promotion of tumor growth and spread.9
About Boehringer Ingelheim in oncology
We have a clear aspiration – to transform the lives of people with cancer by delivering meaningful advances, with the ultimate goal of curing a range of cancers. Boehringer Ingelheim’s generational commitment to driving scientific innovation is reflected by the company’s robust pipeline of cancer cell-directed and immuno-oncology investigational therapies, as well as the smart combination of these approaches. Boehringer’s ambition in oncology is to take a diligent and broad approach, creating a collaborative research network to tap into a diversity of minds, which is vital in addressing some of the most challenging, but potentially most impactful, areas of cancer research. Simply put, for Boehringer Ingelheim, cancer care is personal, today and for generations.
About Boehringer Ingelheim
Boehringer Ingelheim is a biopharmaceutical company active in both human and animal health. As one of the industry’s top investors in Research and Development, the company focuses on developing innovative therapies in areas of high unmet medical need. Independent since its foundation in 1885, Boehringer takes a long-term perspective, embedding sustainability along the entire value chain. More than 53,500 employees serve over 130 markets to build a healthier, more sustainable, and equitable tomorrow. Learn more at www.boehringer-ingelheim.com/us.
References
1Nützinger J, Lee JB, Low JL, et al. Lung Cancer. 2023;186:107385. doi:10.1016/j.lungcan.2023.107385
2International Agency for Research on Cancer – World Health Organization. Rates of trachea, bronchus and lung cancer. Available at: https://gco.iarc.fr/tomorrow/en (Accessed: January 2025).
3Zappa C & Mousa Non-small cell lung cancer: current treatment and future advances, Transl Lung Cancer Res. 2016 Jun; 5(3): 288–300.
4American Cancer Society. Signs and Symptoms of Lung Cancer Available at: https://www.cancer.org/cancer/lung-cancer/detection-diagnosis-staging/signs-symptoms.html (Accessed: January 2025).
5Casal-Mouriño, A. et al. Epidemiology of stage III lung cancer: frequency, diagnostic characteristics, and survival. Transl Lung Cancer Res. 2021;10(1):506-518.
6National Cancer Institute Surveillance, Epidemiology, and End Results (SEER). https://seer.cancer.gov/statfacts/html/lungb.html (Accessed: January 2025).
7Baraibar I, et al. Novel drugs targeting EGFR and HER2 exon 20 mutations in metastatic NSCLC. Crit Rev Oncol Hematol. 2020;148:102906.
8Li, B.T. et al. Trastuzumab Deruxtecan in HER2-Mutant Non–Small-Cell Lung Cancer. N Engl J Med. 2022;386:241–51.
9Galogre M, et al. A review of HER2 overexpression and somatic mutations in cancers, Critical Reviews in Oncology/Hematology, Volume 186, 2023, 103997
Attachment
- Laboratory