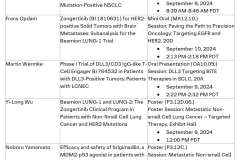
- Beamion LUNG-1, Phase Ib met its primary endpoint, demonstrating a meaningful objective response rate (ORR) of 66.7%, as assessed by blinded independent central review (BICR)1
- Preliminary activity in patients with brain metastases were also observed, with an intracranial response of 33% and 74% disease control rate (DCR)
- Zongertinib was generally well tolerated, with mostly mild and manageable treatment related adverse events (TRAE) and low discontinuation rate due to toxicity (3%)
- With two thirds of patients still on treatment at data cut-off, more mature data including progression-free survival (PFS), and duration of response (DoR) will be reported later in the year
Boehringer Ingelheim reports positive results from a Phase Ib primary analysis of Cohort 1 of the Beamion LUNG-1 trial evaluating zongertinib (BI 1810631) in pre-treated patients with advanced non-small cell lung cancer (NSCLC) with activating HER2 mutations. Zongertinib demonstrated a meaningful objective response rate and was generally well tolerated in the Cohort 1 setting. The results were presented in a Presidential Symposium at the IASLC 2024 World Conference on Lung Cancer (WCLC) and are included in the official 2024 WCLC Press Program.
As of May 2024, 132 patients have been treated with 120 mg / 240 mg of zongertinib once a day (n=75/n=57). With a confirmed objective response rate (ORR) of 66.7%, 97.5% CI (53.8–77.5), (p<0.0001) as assessed by blinded independent central review (BICR), the primary endpoint was met for Cohort 1 (120 mg; n=75). Tumor shrinkage of any magnitude was observed in 94% of all patients across doses, per investigator assessment. The trial design includes a dose expansion that was carried out to find the optimal dose of zongertinib for this patient population. Patients were randomized 1:1 to either the 120 mg (n=58) or the 240 mg (n=55) group. After an interim futility analysis, 120 mg was selected as the dose to be evaluated further in Cohort 1, and 17 additional patients were enrolled. In the part of the trial where patients were randomized 1:1, zongertinib showed a response rate of 72.4% in patients treated with 120 mg daily, and 78.2% in patients treated with 240 mg daily as well as disease control rates (DCRs) of 95% and 100% respectively.
Phase Ib, Cohort 1 data also shows preliminary brain activity with zongertinib. 33% (120 mg; n=27) and 40% (240 mg; n =25) of patients with asymptomatic brain metastases achieved confirmed objective response, with a DCR of 74% and 92% respectively, as per RANO-BM (recommendations for standardized tumor response and progression assessment) by BICR. The central nervous system is a common site of metastasis in NSCLC and is associated with poor prognosis and quality of life.2 Brain metastases are present in up to 30% of patients with NSCLC with activating HER2 mutations at diagnosis.3
'These new data could represent positive news in the future treatment of non-small cell lung cancer patients with activating HER2 mutations,' said the trial’s principal investigator, Dr. John Heymach, MD, PhD, The University of Texas MD Anderson Cancer Center. 'While these mutations are rare, they are critical drivers in a subset of non-small cell lung cancer cases, and current treatment options are severely limited. Patients with this type of cancer typically face a poor prognosis, with approximately 50% responding to first-line treatment and only 20% responding to second-line therapy.”4,5,6,7
Zongertinib is an investigational oral HER2 tyrosine kinase inhibitor (TKI) in development being investigated in patients with advanced NSCLC with activating HER2 mutations. Zongertinib was designed to spare wild-type EGFR thereby mitigating associated toxicities. Beamion LUNG-2, a global Phase III trial evaluating zongertinib compared to standard of care as first-line treatment in patients with advanced NSCLC with activating HER2 mutations is currently enrolling.
Paola Casarosa, Board of Managing Directors, Head of Innovation Unit at Boehringer Ingelheim, said: “Zongertinib’s efficacy and tolerability profile has the potential to become part of the future treatment landscape for patients with HER2 mutated lung tumors. Zongertinib is a perfect example of our approach to science in the discovery and development of novel treatments. Boehringer is committed to providing breakthrough therapies for cancer patients, and we look forward to advancing the zongertinib clinical program.”
Zongertinib was generally well tolerated for 120 mg and 240 mg, with no deaths attributed to treatment and a low incidence of adverse events leading to dose reductions (11%) and discontinuation (3%). No new safety signals or treatment-related interstitial lung diseases (ILD) were observed, and Grade 3 or higher treatment-related adverse events (TRAEs) occurred in 17% (120 mg) and 19% (240 mg) of patients treated with zongertinib. The most common TRAEs were Grade 1 or 2 diarrhea (43% and 11% respectively), Grade 1 or 2 rash (19% and 8% respectively).
Data are still maturing and with two thirds of responding patients still on treatment at data cut-off, progression-free survival (PFS) and duration of response (DoR) data will be reported at an upcoming conference.
About non-small cell lung cancer (NSCLC)
Lung cancer claims more lives than any other cancer type and the incidence is set to increase to over 3 million cases worldwide by 2040.8 NSCLC is the most common type of lung cancer.9 The condition is often diagnosed at a late stage,10 and fewer than 3 in 10 patients are alive five years after diagnosis.11 People living with advanced NSCLC can experience a detrimental physical, psychological, and emotional impact on their daily lives. There remains a high unmet need for additional treatment options for people living with advanced NSCLC. Up to 4% of lung cancers are driven by HER2 mutations (or gene alterations).12 Mutations in HER2 can lead to overexpression and overactivation, which can in turn result in uncontrolled cell production, inhibition of cell death and promotion of tumor growth and spread.13
About zongertinib
Zongertinib (also known as BI 1810631) is an investigational oral HER2-specific tyrosine kinase inhibitor (TKI) that is being developed as a potential treatment for HER2 mutated non-small cell lung cancer (NSCLC). Zongertinib was granted FDA Fast Track Designation in 2023, then in 2024 it was granted Breakthrough Therapy Designation by the U.S. FDA and China CDE for the treatment of adult patients with advanced NSCLC whose tumors have activating HER2 mutations, and who have received a prior systemic therapy. HER2 is a member of the ErbB family of receptor tyrosine kinases (enzymes that act like chemical messengers).14 A recent study has shown pre-clinically that the investigational compound zongertinib has potential for further clinical study in HER2 dependent solid cancers as monotherapy and as concurrent treatment with ADC therapy or with KRAS-targeted drugs.14
About the Beamion clinical trial program
Beamion LUNG-1 (NCT04886804): An open-label, Phase I dose escalation trial, with dose confirmation and expansion, of zongertinib as monotherapy in people with advanced or metastatic solid tumors and NSCLC with activating HER2 mutations. The study has 2 parts. The first part is open to adults with different types of advanced cancer (solid tumors with changes in the HER2 gene) for whom previous treatment was not successful. The second part is open to people with non-small cell lung cancer with a specific mutation in the HER2 gene. Beamion LUNG-2 is a phase 3, open label, randomized, active-controlled study that will enroll 270 patients with unresectable, locally advanced or metastatic non-squamous NSCLC harboring HER2 tyrosine kinase domain mutations to evaluate Zongertinib compared with standard of care.
About Boehringer Ingelheim in oncology
We have a clear aspiration – to transform the lives of people with cancer by delivering meaningful advances, with the ultimate goal of curing a range of cancers. Boehringer Ingelheim’s generational commitment to driving scientific innovation is reflected by the company’s robust pipeline of cancer cell-directed and immuno-oncology investigational therapies, as well as the smart combination of these approaches. Boehringer’s ambition in oncology is to take a diligent and broad approach, creating a collaborative research network to tap into a diversity of minds, which is vital in addressing some of the most challenging, but potentially most impactful, areas of cancer research. Simply put, for Boehringer Ingelheim, cancer care is personal, today and for generations.
About Boehringer Ingelheim
Boehringer Ingelheim is a biopharmaceutical company active in both human and animal health. As one of the industry’s top investors in Research and Development, the company focuses on developing innovative therapies in areas of high unmet medical need. Independent since its foundation in 1885, Boehringer takes a long-term perspective, embedding sustainability along the entire value chain. More than 53,500 employees serve over 130 markets to build a healthier, more sustainable, and equitable tomorrow.
References
1Ruiter G. et al. Phase Ib Analysis of Beamion LUNG-1: Zongertinib (BI 1810631) in Patients with HER2-Mutant NSCLC. presented at WCLC, San Diego, 7-10 September, 2024.
2Arrieta, O., Saavedra-Perez, D., Kuri, R. et al. Brain metastasis development and poor survival associated with carcinoembryonic antigen (CEA) level in advanced non-small cell lung cancer: a prospective analysis. BMC Cancer 9, 119 (2009). https://doi.org/10.1186/1471-2407-9-119.
3Offin M, et al. Cancer 2019;125(24):4380–87.
4Nützinger J, Lee JB, Low JL, et al. Lung Cancer. 2023;186:107385. doi:10.1016/j.lungcan.2023.107385
5Brazel D, Kroening G, Nagasaka M. BioDrugs. 2022;36(6):717-729.
6Jebbink M, de Langen AJ, Boelens MC, Monkhorst K, Smit EF. Cancer Treat Rev. 2020;86:101996. doi:10.1016/j.ctrv.2020.101996
7Passaro A, Peters S. N Engl J Med. 2022;386(3):286-289.
8International Agency for Research on Cancer – World Health Organization. Rates of trachea, bronchus and lung cancer. Available at: https://gco.iarc.fr/tomorrow/en (Accessed August 2024).
9Zappa C & Mousa Non-small cell lung cancer: current treatment and future advances, Transl Lung Cancer Res. 2016 Jun; 5(3): 288–300.
10Polanco D et al. Prognostic value of symptoms at lung cancer diagnosis: a three-year observational study. J Thorac Dis 2021;13:1485–1494
11National Cancer Institute Surveillance, Epidemiology, and End Results (SEER). https://seer.cancer.gov/statfacts/html/lungb.html (Accessed: August 2024).
12Arcila, M. E. et al. Prevalence, clinicopathologic associations, and molecular spectrum of ERBB2 (HER2) tyrosine kinase mutations in lung adenocarcinomas. Clin. cancer Res. an Off. J. Am. Assoc. Cancer Res. 18, 4910–4918 (2012).
13Galogre M, et al. A review of HER2 overexpression and somatic mutations in cancers, Critical Reviews in Oncology/Hematology, Volume 186, 2023, 103997
14Wilding, B et al. Cancer Discov. 2024. DOI 10.1158/2159-8290.CD-24-0306
Attachment
- Boehringer laboratory